Evolutionary biochemistry of Biliverdin Binding Serpins (BBS): biochemical tricks that create leaf-like greens
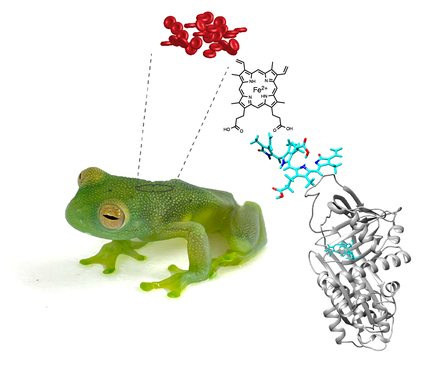
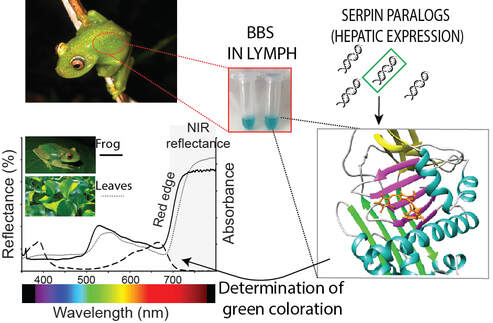
Leaf-like treefrog species from all over the world are characterized by skin reflectances that perfectly match that of the leaves where they sleep, both in the visible portion of the spectrum, and in the near-infrared (NIR). Our work has shown that green coloration in treefrogs arose multiple times in the evolutionary history of amphibians. Yet, each time, green coloration depended on the same biochemical trick: the expression of colored proteins of the serpin superfamily.
Serin Protease Inhibitors (Serpin) are the largest superfamily of protease inhibitors, and they are known for their dramatic conformational change to inhibit target proteases. Serpins are ubiquitous in all organisms, and in vertebrates they are key players in the regulation of blood coagulation and inflammation. We found that during the evolutionary history of frogs, diverse clades have co-opted at least one member of the serpin group, and evolved a different set of amino acid substitutions that allow them to bind biliverdin. These biliverdin-binding serpins (BBSs) produce a color change that approximates the optical properties of plant pigments such as chlorophyll and phytochromes. With more than 40 independent origins, the use of BBSs for the creation of green coloration is one of the most striking examples of convergence in nature.
In the lab, we use biochemical methods to purify, sequence, and characterize the biophysical properties of BBSs. We are interested in how the evolution of their sequences led to various binding mechanisms to biliverdin. and how the local changes in the binding pockets can finely tune the light absorption properties of BBSs, which ultimately creates different green colors in treefrogs. We currently have a large dataset of BBSs from multiple independent origins including Hylids, Centrolenids, Hyperolids and Mantellids. Given the complexity of the sequence space (different BBSs have ~50% identity) we implement an evolutionary biochemistry framework to reconstruct ancestral proteins, experimentally express those ancestors, and elucidate the sequence of changes that led to the biophysical properties of each BBS.
In our research, we use biochemical, biophysical and evolutionary biology methods, including the expression of recombinant proteins, purification of natural variants or isoforms, fluorescence spectroscopy, mass spectrometry, isothermal titration calorimetry, circular dichroism, NIR imaging, transcriptomics and various methods to measure and simulate light propagation and the creation of colors.
|
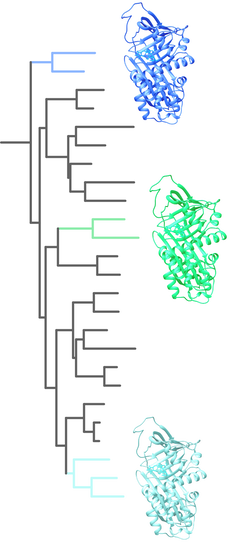
BBSs arose multiple times along the evolutionary history of frogs.
All of them create the leaf-like coloration of treefrogs but rely on different biophysical properties of BBSs that were tuned by aminoacid substitutions tuned over millions of years. BBSs from different species have only 40-75% identity, which for a protein of 400 amino acids means that roughly ~200 amino acids in the BBSs structure are conserved between them. An evolutionary biochemistry approach allows us to investigate the changes in the sequence space that allowed the biochemical changes to bind biliverdin and ultimately produce colors. |